| This article was first published in The
Chemical Intelligencer, July, 1995 (Vol. 1, No. 3), edited by Istvan
Hargittai (Institute of General and Analytical Chemistry, Budapest Technical
University) and published by Springer-Verlag New York Inc. The picture
of EJA with RBF (credit card in hand) appeared in the original, along
with the small portrait at the end. Other artwork and hyperlinks were
added for this version. The text is unchanged from the original. Reproduced
with EJA's permission. The BFI much later created its
own reproduction.
| |||
The Naming of Buckminsterfullerene | |||
|
The Israeli poet Yehuda Amichai once described naming as "the primary cultural activity," the crucial first step anyone must take before embarking on thought. John Stuart Mill declared that "The tendency has always been strong to believe that whatever received a name must be an entity or being, having an independent existence of its own." |

| ||
|
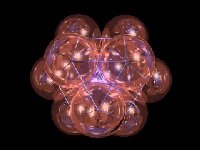
When Harry Kroto and Richard Smalley, the experimental
chemists who discovered
|
| ||
|
This newly discovered molecule, a third allotrope
of carbon - ancient and ubiquitous - transcends the historical
or geographical significance of most named phenomena such as mountains
of the moon or Antarctic peaks and ridges. Cartographers named
two continents for Amerigo Vespucci, because he asserted (as Columbus
did not) that the coasts of Brazil and the islands of the Caribbean
were a landmass of their own and not just obstacles on the route
to Asia. |
|||
|
Buckminsterfullerene was discovered by chemists who
were not looking for what they found. Kroto was looking for an
interstellar molecule. Smalley said he hadn't been very interested
in soot, but they agreed to collaborate. Smalley's laboratory
at Rice University had the exquisite laser-vaporization and mass
spectrometry equipment to describe the atoms of newly created
molecules. Scientific experimenters investigate nature at a level
where revelation is often unpredictable and sometimes capricious.
This is a phenomenon that Fuller (who was not a scientist, but
a staunch defender of the scientific method) generalized into
the dogmatic statement that all true discovery is precessional.
For Fuller, the escape from accepted paradigms is precessional.
(Vespucci precessed; Columbus did not.) Fuller had a lifelong
preoccupation with the counter-intuitive, gyroscopic phenomenon
of precession. He defined precession, quite broadly, as the effect
of bodies in motion on other bodies in motion. Every time you
take a step, he said to me many times, you precess the universe.
|
| ||
|
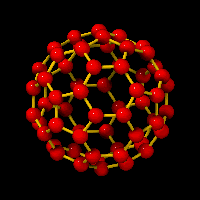
For that matter, one may say that Kroto and Smalley,
in recognizing the shape of the |
| ||
|
As a longtime close friend of the Fuller family,
as his collaborator on his two volumes of Synergetics (1975,
1979), and as a trustee of the Buckminster Fuller Institute (BFI),
I rejoiced vicariously in the molecular celebration of his name.
I preserved the copy of its first publication in Nature (November
1985), with the |
Buckminster Fuller and E.J. Applewhite at a midnight supper at the Waldorf-Astoria Hotel celebrating their completion of the final galleys of Synergetics. Fuller in his own hand has inscribed the mat of this photograph as follows: "Entering the home stretch of the 1/2-century long, Synergetics galley race. |
||
|
Although I felt that it was presumptuous for me,
as a nonscientist, to address Kroto and Smalley on Fuller's behalf,
I nevertheless offered them copies of Fuller's Synergetics
books and drew their attention to collateral aspects of Fuller's
work that might be relevant to their major discovery. I was careful
to disavow any claim for priority of discovery on Fuller's behalf.
He did not anticipate |
| ||
|
Some years later, on March 21, 1991, on a visit to
Houston, I had the opportunity to call on Professor Smalley in
his laboratory at Rice University and pay him homage, specifically
on behalf of the Fuller family and the BFI - expressing our gratification
in the luster that he and Professor Kroto had added to Fuller's
name. He greeted me with a hospitality, a sympathy, and an enthusiasm
matching the cordiality of the correspondence I had initiated
with Professor Kroto at the University of Sussex in Brighton.
A sense of destiny permeates his large, comfortable office; he
told me I was sitting on the very couch where he and Kroto christened
the new molecule on September 9, 1985. He told me about how he
and his colleagues had sat up all night making models out of Gummy
Bear jelly beans and paper cutouts of pentagons and hexagons.
I recalled that Fuller as a child had made models out of toothpicks
and dried peas, and he had always felt that geometry should be
taught as a hands-on laboratory discipline. Smalley said that
he had overcome any initial reservations he might have had to
Kroto's proposal to name |

| ||
|
As Smalley escorted me out of the laboratory complex
on that steaming hot March afternoon (Houston is like that), I
was exhilarated by his conviction that After a few letters objecting to the name buckminsterfullerene had appeared in the columns of Nature, Harry Kroto gallantly defended its choice on the grounds that no other name - none of the forms of the classic Greek geometers - described the essential three properties of lightness, strength, and the internal cavity that the geodesic dome affords. To the protest that nobody had ever heard of Fuller, he submitted that the name would have educational value. A fine exercise of onomastic prerogative. |
|
||
| Fuller was not a chemist. He was not
even a scientist, and made no pretension of adhering rigidly to an experimental
and deductive methodology, and he did not follow the rules of submitting
published papers to peer review. But he had an extraordinary facility
for intuitive conceptioning. Jim Baggott, in his superb account Perfect
Symmetry: The Accidental Discovery of Buckminsterfullerene [2]
quotes Fuller in an epigraph: "Are there in nature behaviors of whole
systems unpredicted by the parts? This is exactly what the chemist has
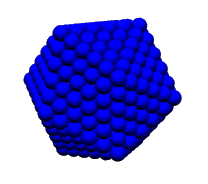
discovered to be true." Baggott goes on to describe how Fuller had
derived his vector equilibrium (cuboctahedron, in conventional geometry)
from the closest packing of spheres of energy. What he had was a principle
that led to the design of geodesic structures capable of a strength-to-weight
ratio impossible in more conventional structures. Fuller had a highly
generalized definition of the function of architecture that put him outside
the scope of the academicians' view of their discipline. Bucky said "architecture
is the making of macrostructures out of microstructures."
Baggott concludes: "Fuller's thoughts about the patterns of forces in structures formed from energy spheres had led him to the geodesic domes.... That his geodesic domes should serve as a basis for rediscovering these principles in the context of a new form of carbon microstructure has a certain symmetry that Fuller would have found pleasing, if not very surprising." |
| ||
REFERENCES | |||
 E. J. APPLEWHITE grew up in Newport News, Virginia,
except for two years spent in Tahiti. He graduated from Yale University
in 1941 and later went to the Harvard Business School. After Navy
service on an aircraft carrier, he worked with Fuller in a housing
project in Wichita, Kansas. He joined the CIA in 1947 and served
in Bonn and Beirut. In 1979 he completed nine years of collaboration
with Fuller on his Synergetics books. He describes himself as a layman.
E. J. APPLEWHITE grew up in Newport News, Virginia,
except for two years spent in Tahiti. He graduated from Yale University
in 1941 and later went to the Harvard Business School. After Navy
service on an aircraft carrier, he worked with Fuller in a housing
project in Wichita, Kansas. He joined the CIA in 1947 and served
in Bonn and Beirut. In 1979 he completed nine years of collaboration
with Fuller on his Synergetics books. He describes himself as a layman.
| |||